*/
Robotic surgery is something of a misnomer. What it really means, at least for now, is human surgeons performing operations using robotically assisted tools that are entirely under their control.
These tools broadly fit into two distinct categories. The first is telemanipulation systems, where the surgeon sits at a console and uses a hand controller to move the instruments inside the patient. These robots have multiple arms – usually three to hold surgical instruments, and a fourth for the 3D camera that allows the surgeon to see what they are doing. The most widely used are the da Vinci models from Intuitive, based in California. However, competitors have entered the market more recently – including the British-built Versius from CMR Surgical. Telemanipulation systems are generally used for soft tissue procedures in specialities like urology, gynaecology, and colorectal.
The second category of surgical robot is ‘assistive guide’ systems, such as Stryker’s Mako. Predominantly used for orthopaedic and neurosurgery procedures, these platforms consist of a single robotic arm combined with a navigation system. The surgeon creates a preoperative plan based on scans of the patient’s anatomy and then operates on the patient directly, using the robotic arm to ensure that they execute their plan with a high degree of accuracy.
The first da Vinci was introduced in 1999, and today there are more than 6,700 installed worldwide. By the end of 2021, surgeons had performed a cumulative 10 million operations using da Vinci robots alone. Dozens of other companies have either already launched their own systems, or plan to soon, and adoption of these technologies seems set to continue at an ever-increasing rate. The features of the robotic platforms vary by brand and model, but the most cited reason for their adoption is that they make it simpler for surgeons to learn and perform complex minimally invasive surgeries. These procedures involve smaller incisions than open surgery, and can mean a faster and more painless recovery for patients postoperatively.
The future of robotics in surgery is likely to be less about the physical robots themselves than what they enable. One thing that all the platforms have in common is the powerful computers they contain for control of their electromechanical components. By introducing robots to operating theatres, we are therefore also putting a computer in the room, between the surgeon and the patient, for the first time. This is an opportunity to move surgery from subjective artistry to something that can be quantified and improved at scale using software. To see why this might be significant for both clinical and legal practice, it is worth considering some features that this shift might enable.
The first is the concept of surgical ‘flight recorders’ inspired by black boxes in commercial aviation. When something goes wrong in surgery today, events must be reconstructed from participants memories and notes. With a surgical flight recorder, it would be possible to simply review the data from the relevant portion of the procedure. Surgical robots can already produce telemetry data precisely recording the surgeons hand movements and resultant instrument paths. They also have the video feed from the surgical field, showing the surgeon’s view. In this scenario, it would be possible to see exactly what the robotic instruments were doing at the time of the incident, as well as the movements of the surgeon, what they could see, and how they responded.
Another area of significant research interest is using machine vision technologies to analyse surgical video from the procedure in real time to provide safety information and interventions to the surgeon. A good example of this is AI-enabled identification of critical anatomical structures, such as major blood vessels or the ureters. The onboard software could then create ‘no-fly’ zones that prevent the robotic instruments getting too close to those structures, reducing the risk of accidental damage. This technology is still some way off being used outside of trials, but multiple companies are known to be making progress towards commercially viable products in this space.
The future of robotics in surgery is therefore likely to be smarter than the fully surgeon-controlled systems that we see in use today. As these advanced safety capabilities become available, they will raise interesting questions about when and how they should be used. If they are shown to work, however, then perhaps the most challenging questions will be under what circumstances is it clinically justifiable not to use them.
Pushing boundaries, in any sphere, brings risk. When that sphere is healthcare, unmanaged risk may lead to injury or even death to vulnerable patients. Mechanical failure, component malfunction and electric arcing are risks associated with robot-assisted surgery. But basic organisational and surgical errors occur too.
There are no reported cases in this jurisdiction dealing with civil liability but some of the issues were highlighted in the inquest touching on the death of Stephen Pettitt, who died in February 2015 after robot-assisted heart surgery. The inquest heard that the lead surgeon had received no prior one-to-one training and had only practised on a simulator. He had only observed four relevant robotic operations. Mr Pettitt’s operation was the first of its kind in the UK. The surgeon had arranged the salient aspects of the procedure himself, with little or no oversight.
The coroner found that there were no relevant guidelines in place regarding the training requirements for the procedure, the recruitment and use of experienced surgeons to oversee the surgeon performing the operation or the advice to be provided to patients. The Royal College of Surgeons has acknowledged that there are few regulatory requirements for innovative surgery. However, they have issued no formal guidelines in direct response to the coroner’s concerns.
The NHS has more than 60 robotic surgery machines in use. It is expected that they will perform a crucial role in reducing the backlog caused by the pandemic. In January 2022, in the USA, a surgical robot operated free from human control, for the first time, on a pig. Can broad industry guidelines and historic government regulation adapt to recent innovations in robotics and the advance of AI? Does the legal system have the tools to protect patients? Liability for failure or poor outcomes may be unclear.
General principles in civil litigation in tort (medical negligence) and actions for defective products (under The Consumer Protection Act 1987) should provide adequate redress, even if the cases themselves have complicated and unusual characteristics. Identifying the correct defendant may be problematic but robots/computers store metadata which can lead to the answer, as the data can form a sort of digital footprint of what the surgeon did and how the machine responded.
One area that might need considerable focus from litigators and surgeons is informed consent. In Montgomery v Lanarkshire Health Board [2015] UKSC 11 the Supreme Court held that obtaining informed consent would require the surgeon to ensure that the patient was aware of any (patient specific) material risks and of other reasonable treatment options. If robot-assisted surgery is planned, there is likely to be an obligation to ensure the patient is informed about alternative conventional options, the relative advantages of each approach, the training the surgeon has undergone, whether someone from the technology company (or another qualified surgeon) will be present and what procedures are in place if a conversion to conventional surgery is required.
Advances in AI will lead to greater robotic autonomy. There are proposals for regulation in Europe. The EU produced a detailed study in 2016 and a comprehensive industrial policy on AI and robotics in 2019. The Commission has since drafted a white paper on artificial intelligence and a draft report with recommendations on a civil liability regime. At one stage, legal person status for robots was considered but discarded for strict liability and compulsory insurance. The EU concluded that the current product safety legislation contained serious gaps. Westminster produced discussion papers aptly titled AI in the UK: No Room for Complacency and, separately, passed a compulsory insurance scheme with strict liability for driverless cars. One could envisage a similar regime being appropriate for robots in healthcare but, to date, no formal legislation has been proposed.
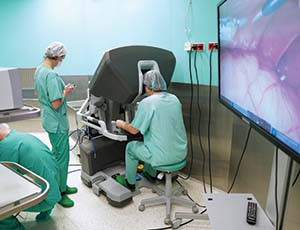
Pictured above: bypassing the coronary arteries without opening the patient’s chest, using the ‘Da Vinci’ robot, at the Central Clinical Hospital of the Ministry of Interior and Administration at Woloska Street in Warsaw, Poland, 15 February 2021.

Robotic surgery is something of a misnomer. What it really means, at least for now, is human surgeons performing operations using robotically assisted tools that are entirely under their control.
These tools broadly fit into two distinct categories. The first is telemanipulation systems, where the surgeon sits at a console and uses a hand controller to move the instruments inside the patient. These robots have multiple arms – usually three to hold surgical instruments, and a fourth for the 3D camera that allows the surgeon to see what they are doing. The most widely used are the da Vinci models from Intuitive, based in California. However, competitors have entered the market more recently – including the British-built Versius from CMR Surgical. Telemanipulation systems are generally used for soft tissue procedures in specialities like urology, gynaecology, and colorectal.
The second category of surgical robot is ‘assistive guide’ systems, such as Stryker’s Mako. Predominantly used for orthopaedic and neurosurgery procedures, these platforms consist of a single robotic arm combined with a navigation system. The surgeon creates a preoperative plan based on scans of the patient’s anatomy and then operates on the patient directly, using the robotic arm to ensure that they execute their plan with a high degree of accuracy.
The first da Vinci was introduced in 1999, and today there are more than 6,700 installed worldwide. By the end of 2021, surgeons had performed a cumulative 10 million operations using da Vinci robots alone. Dozens of other companies have either already launched their own systems, or plan to soon, and adoption of these technologies seems set to continue at an ever-increasing rate. The features of the robotic platforms vary by brand and model, but the most cited reason for their adoption is that they make it simpler for surgeons to learn and perform complex minimally invasive surgeries. These procedures involve smaller incisions than open surgery, and can mean a faster and more painless recovery for patients postoperatively.
The future of robotics in surgery is likely to be less about the physical robots themselves than what they enable. One thing that all the platforms have in common is the powerful computers they contain for control of their electromechanical components. By introducing robots to operating theatres, we are therefore also putting a computer in the room, between the surgeon and the patient, for the first time. This is an opportunity to move surgery from subjective artistry to something that can be quantified and improved at scale using software. To see why this might be significant for both clinical and legal practice, it is worth considering some features that this shift might enable.
The first is the concept of surgical ‘flight recorders’ inspired by black boxes in commercial aviation. When something goes wrong in surgery today, events must be reconstructed from participants memories and notes. With a surgical flight recorder, it would be possible to simply review the data from the relevant portion of the procedure. Surgical robots can already produce telemetry data precisely recording the surgeons hand movements and resultant instrument paths. They also have the video feed from the surgical field, showing the surgeon’s view. In this scenario, it would be possible to see exactly what the robotic instruments were doing at the time of the incident, as well as the movements of the surgeon, what they could see, and how they responded.
Another area of significant research interest is using machine vision technologies to analyse surgical video from the procedure in real time to provide safety information and interventions to the surgeon. A good example of this is AI-enabled identification of critical anatomical structures, such as major blood vessels or the ureters. The onboard software could then create ‘no-fly’ zones that prevent the robotic instruments getting too close to those structures, reducing the risk of accidental damage. This technology is still some way off being used outside of trials, but multiple companies are known to be making progress towards commercially viable products in this space.
The future of robotics in surgery is therefore likely to be smarter than the fully surgeon-controlled systems that we see in use today. As these advanced safety capabilities become available, they will raise interesting questions about when and how they should be used. If they are shown to work, however, then perhaps the most challenging questions will be under what circumstances is it clinically justifiable not to use them.
Pushing boundaries, in any sphere, brings risk. When that sphere is healthcare, unmanaged risk may lead to injury or even death to vulnerable patients. Mechanical failure, component malfunction and electric arcing are risks associated with robot-assisted surgery. But basic organisational and surgical errors occur too.
There are no reported cases in this jurisdiction dealing with civil liability but some of the issues were highlighted in the inquest touching on the death of Stephen Pettitt, who died in February 2015 after robot-assisted heart surgery. The inquest heard that the lead surgeon had received no prior one-to-one training and had only practised on a simulator. He had only observed four relevant robotic operations. Mr Pettitt’s operation was the first of its kind in the UK. The surgeon had arranged the salient aspects of the procedure himself, with little or no oversight.
The coroner found that there were no relevant guidelines in place regarding the training requirements for the procedure, the recruitment and use of experienced surgeons to oversee the surgeon performing the operation or the advice to be provided to patients. The Royal College of Surgeons has acknowledged that there are few regulatory requirements for innovative surgery. However, they have issued no formal guidelines in direct response to the coroner’s concerns.
The NHS has more than 60 robotic surgery machines in use. It is expected that they will perform a crucial role in reducing the backlog caused by the pandemic. In January 2022, in the USA, a surgical robot operated free from human control, for the first time, on a pig. Can broad industry guidelines and historic government regulation adapt to recent innovations in robotics and the advance of AI? Does the legal system have the tools to protect patients? Liability for failure or poor outcomes may be unclear.
General principles in civil litigation in tort (medical negligence) and actions for defective products (under The Consumer Protection Act 1987) should provide adequate redress, even if the cases themselves have complicated and unusual characteristics. Identifying the correct defendant may be problematic but robots/computers store metadata which can lead to the answer, as the data can form a sort of digital footprint of what the surgeon did and how the machine responded.
One area that might need considerable focus from litigators and surgeons is informed consent. In Montgomery v Lanarkshire Health Board [2015] UKSC 11 the Supreme Court held that obtaining informed consent would require the surgeon to ensure that the patient was aware of any (patient specific) material risks and of other reasonable treatment options. If robot-assisted surgery is planned, there is likely to be an obligation to ensure the patient is informed about alternative conventional options, the relative advantages of each approach, the training the surgeon has undergone, whether someone from the technology company (or another qualified surgeon) will be present and what procedures are in place if a conversion to conventional surgery is required.
Advances in AI will lead to greater robotic autonomy. There are proposals for regulation in Europe. The EU produced a detailed study in 2016 and a comprehensive industrial policy on AI and robotics in 2019. The Commission has since drafted a white paper on artificial intelligence and a draft report with recommendations on a civil liability regime. At one stage, legal person status for robots was considered but discarded for strict liability and compulsory insurance. The EU concluded that the current product safety legislation contained serious gaps. Westminster produced discussion papers aptly titled AI in the UK: No Room for Complacency and, separately, passed a compulsory insurance scheme with strict liability for driverless cars. One could envisage a similar regime being appropriate for robots in healthcare but, to date, no formal legislation has been proposed.

Pictured above: bypassing the coronary arteries without opening the patient’s chest, using the ‘Da Vinci’ robot, at the Central Clinical Hospital of the Ministry of Interior and Administration at Woloska Street in Warsaw, Poland, 15 February 2021.


The Bar Council is ready to support a turn to the efficiencies that will make a difference
A £500 donation from AlphaBiolabs has been made to the leading UK charity tackling international parental child abduction and the movement of children across international borders
By Louise Crush of Westgate Wealth Management
Marie Law, Director of Toxicology at AlphaBiolabs, examines the latest ONS data on drug misuse and its implications for toxicology testing in family law cases
An interview with Rob Wagg, CEO of New Park Court Chambers
What meaningful steps can you take in 2026 to advance your legal career? asks Thomas Cowan of St Pauls Chambers
Ever wondered what a pupillage is like at the CPS? This Q and A provides an insight into the training, experience and next steps
The appointments of 96 new King’s Counsel (also known as silk) are announced today
Ready for the new way to do tax returns? David Southern KC continues his series explaining the impact on barristers. In part 2, a worked example shows the specific practicalities of adapting to the new system
Resolution of the criminal justice crisis does not lie in reheating old ideas that have been roundly rejected before, say Ed Vickers KC, Faras Baloch and Katie Bacon
With pupillage application season under way, Laura Wright reflects on her route to ‘tech barrister’ and offers advice for those aiming at a career at the Bar